This method uses the principle of dosing the sorbent into the flue gas flow.
It is possible to use dry hydrate Ca(OH)2 or sodium bicarbonate NaHCO3as a sorbent. The reactions of SO2 with the sorbent take place primarily in the smoke stacks and they continue, to a lesser extent, in the layer of separated substances created on the filtration hoses of the bag filter. The products of the reaction are solid substances and they are separated, together with fly ash, in the bag filter. The efficiency of desulphurisation depends on the sorbent used. The reduction in emissions of acidic components is controlled through the number of doses of the sorbent.
Chemical reactions of sorbent with SO2
Using Ca(OH)2
Ca(OH)2 + SO2 →CaSO3 * 0,5H2O + 0,5H2O
Using NaHCO3
| Thermic activation – calcination | 2NaHCO3 → Na2CO3 + CO2 + H2O |
|---|---|
| Neutralising reaction | Na2CO3 + SO2 → Na2SO3 + CO2 |
| OxiOxidationdace | Na2SO3 + 0,5O2 → Na2SO4 |



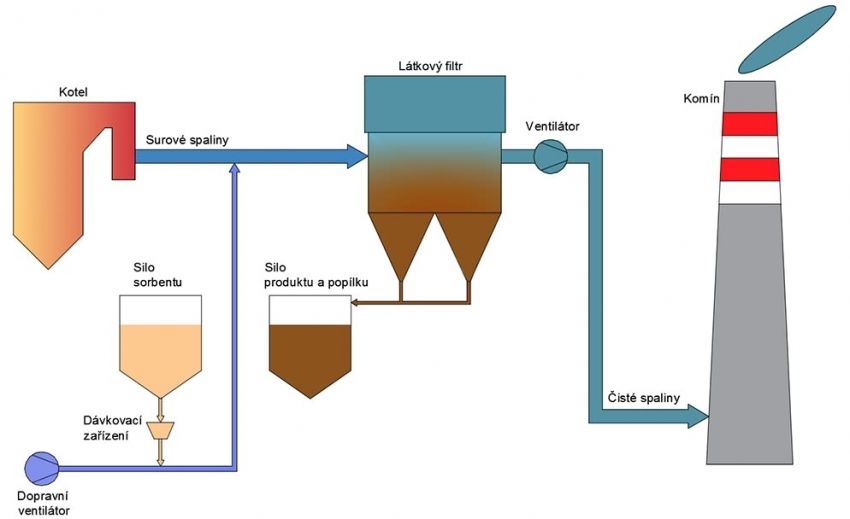
Basic technological units
- sorbent storage and dosing
- bag filter
- transporting the product to the silo
- smoke stacks, flue-gas ventilator
- reactor utilisation option (sorbent Ca(OH)2)
- reactor and water management utilisation option (sorbent Ca(OH)2)
Product handling and utilisation
- utilisation of the product of dry method desulphurisation is problematic and the product is mostly deposited in dumping and disposal sites
- if the Ca(OH)2 sorbentje is used, it is possible to create stabilisate from the resulting product. The main components of the desulphurisation product include CaO, CaCO3, CaSO3 a CaSO4
- if the NaHCO3 sorbent is used, the desulphurisation product is non-utilisable
- main components of the desulphurisation product include NaCl, NaF, Na2SO3 a Na2SO4


Advantages of the method
- simple technology
- low investment costs
- dry process
- short implementation time
- minimum spatial requirements
- minimum interference with the existing technology